Transitioning to ENFit
WHAT IS ENFIT?
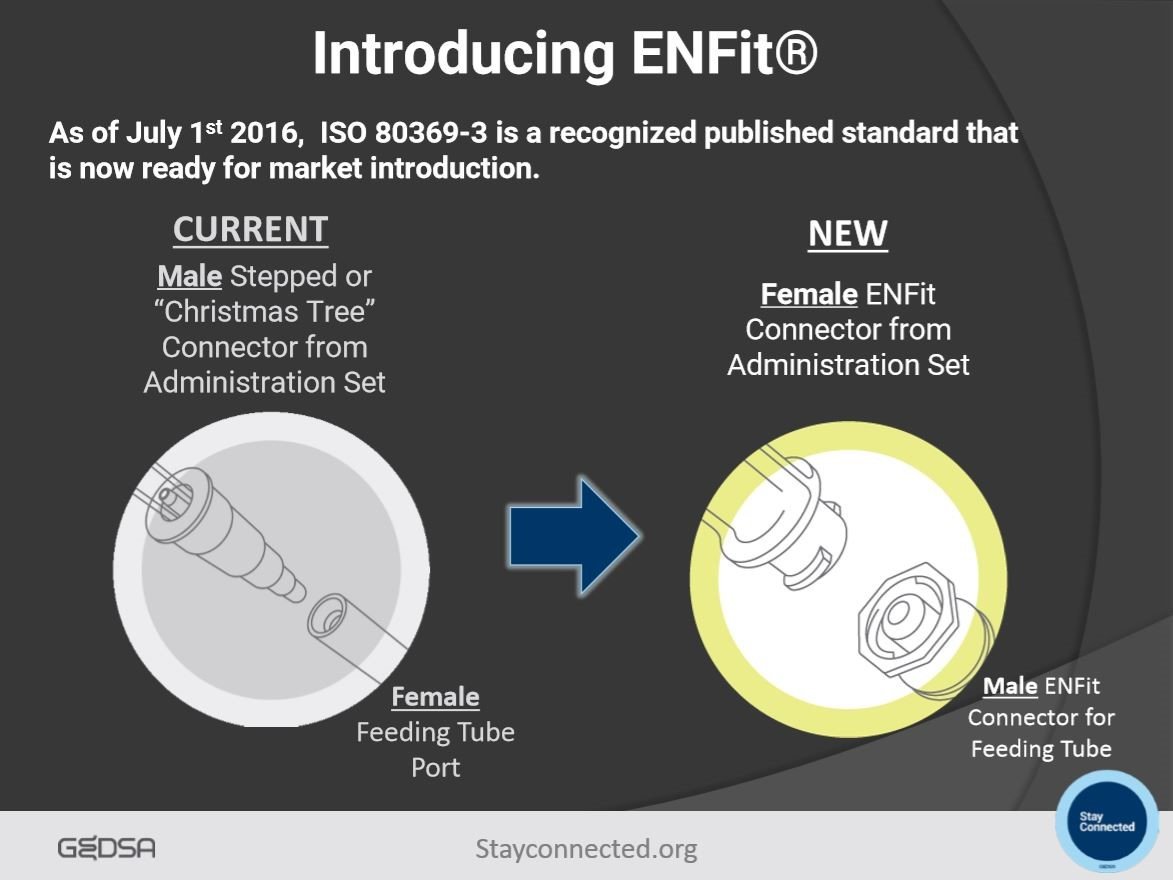
ENFit, or ISO 80369-3, is an Enteral Feeding Connector uniquely designed to reduce the risk of tubing misconnections. This design was created under an international series of standards called ISO 80369. These standards “make it difficult, if not impossible, for unrelated delivery systems to be connected.” Tubing misconnections are an “under-reported error” that highly impacts patient safety. These connection errors occur when one type of medical device is mistakenly attached to another medical device that serves a completely different function; therefore, leading to patient harm or death. ENFit however, is designed specifically for enteral functions and cannot be connected to any other medical device (Shown in Figure 1). ENFit also has a “locking feature that signals the appropriate connection and stays in place” to avoid any disconnections. Transitioning to ENFit is a long process due to the magnitude of change but necessary to ensure patient safety worldwide.
*Figure 1
TRANSITIONING TO ENFIT
The transition to ENFit connectors has already begun and is an on-going process led by the Global Enteral Device Supplier Association (GEDSA). The GEDSA is a group of medical device manufacturers working to increase patient safety by introducing international standards for medical tubing devices. Knowing this would not be an easy task, suppliers designed a temporary transitional connecting device (Shown in Figure 2); solely created to be used until all feeding tubes, pump sets, and syringes have completely transitioned to ENFit. All hospitals and clinicians were encouraged to coordinate with suppliers in order to establish a “go live” date for the new ENFit devices. However, due to COVID-19 and other concerns, the transition to ENFit has been prolonged and the GEDSA revised the ENFit conversion schedule for U.S. and Canada. As of May 6, 2020, many hospital systems have decided that ENFit connectors meet their patient safety requirements and plan to implement these new devices. More recently, hospitals such as Colorado Children’s are fully transitioning over to ENFit and other hospitals have a set “go live” date, including Texas Children’s who is set to convert on October 14, 2020.
*Figure 2
WHAT TO EXPECT
Even though ENFit has been through precise testing and assessments such as “performance testing, misconnections assessment, human factors, computer aid design (CAD), and usability and risk analysis testing”, many concerns have been brought forth during the ENFit implementation process from both the provider and patient side. Most of the patient’s concerns involved using ENFit with blenderized diets. The FDA identified and responded to three significant concerns from G-tube users; clogging, a slow gravity feeding flow, and the need for more force to move food through G-tubes for push-mode users. The FDA conducted more research, specific to blenderized diets, and concluded that “For a push mode of feeding, patients will largely be unimpacted after the transition to ENFit. For a gravity mode of feeding, some ENFit users may need higher-powered blenders and should expect increased feeding times.” So what can home enteral nutrition users expect from this point on? It is no surprise that this conversion will take time. Extensive testing by Mayo Clinic is on-going and “includes various commercial formulas and blenderized food products to ensure that tube feeders’ dietary choices will not be impacted by the move to ENFit.” Medical professionals should be aware of the transition to ENFit due to GEDSA efforts; however, not all people affected are fully aware of all the occurring changes. Those affected are strongly encouraged to educate themselves and develop their personal opinions on ENFit knowing the previous research that has been conducted. Moving forward, it is important to remember that through this process the ultimate goal is patient safety and improving our health care system.
References
https://www.fda.gov/media/115846/download
http://stayconnected.org/enteral-enfit/
https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/medical-device-connectors
http://stayconnected.org/wp-content/uploads/2018/10/GEDSA-Position-Statement-on-FDA-Letter-2018-.pdf
https://www.premiersafetyinstitute.org/safety-topics-az/tubing-misconnections/tubing-misconnections/
http://stayconnected.org/wp-content/uploads/2016/09/ESPEN-Presentation.pdf
https://www.tandfonline.com/eprint/HWc9Ad3grFHFjaPKnI5G/full?target=10.1080%2F07315724.2018.1509247&
Disclaimer
This report does not provide medical advice and is intended for educational purposes only. Please talk to a medical professional if you have any questions regarding ENFit.